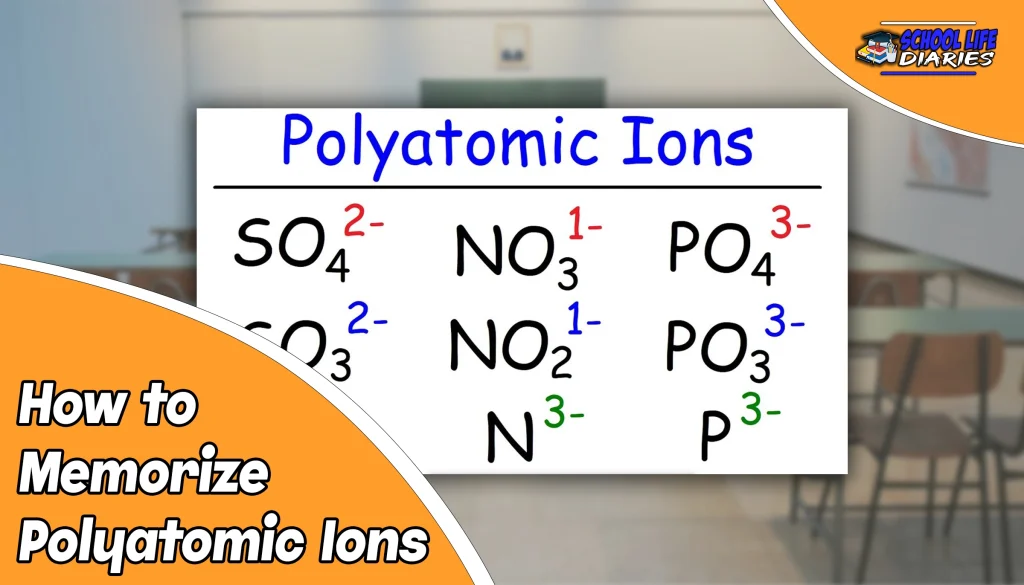
Are you struggling to memorize polyatomic ions? You’re not alone. Many students find it difficult to remember the names and charges of these groups of atoms. But don’t worry, with the right approach, memorizing polyatomic ions can be a breeze.
In this article, we’ll go over everything you need to know to memorize polyatomic ions, from the basics to advanced techniques.
Introduction
Polyatomic ions are groups of atoms that have a net charge. They are commonly found in ionic compounds and play an important role in chemistry. Memorizing the names and charges of these ions can be challenging, especially for beginners.
There are several techniques and tricks that can make the process much easier. Whether you’re a student preparing for an exam or simply interested in chemistry, this guide will help you memorize polyatomic ions quickly and effectively.
How To Memorize Polyatomic Ions?
Learning polyatomic ions can seem daunting at first, but with a bit of effort and practice, you can master them in no time. Here are some steps you can take to make the process easier:
1. Understand the Basics:
Before you start memorizing, it’s important to understand the basics of polyatomic ions. A polyatomic ion is a group of atoms that are covalently bonded together but have a net charge.
The charge comes from the gain or loss of electrons, which creates an imbalance in the number of protons and electrons.
2. Focus on Common Ions:
There are many polyatomic ions, but you don’t need to memorize them all. Focus on the most common ones that you’re likely to encounter. For example, sulfate (SO4 2-) and nitrate (NO3 -) are two of the most common polyatomic ions.
3. Use Mnemonics:
Mnemonics are memory aids that can help you remember information more easily. One popular mnemonic for polyatomic ions is “Oh My, Such Good Apple Pie, So Sweet And Yummy.” Each word in the phrase represents the first letter of a common polyatomic ion: hydroxide (OH-), sulfate (SO4 2-), carbonate (CO3 2-), phosphate (PO4 3-), sulfite (SO3 2-), and ammonium (NH4 +).
4. Create Flashcards:
Flashcards are a great way to memorize polyatomic ions. Write the name of the ion on one side of the card and the charge on the other. Test yourself regularly and shuffle the cards to keep it challenging.
5. Practice with Quizlet:
Quizlet is a free online tool that allows you to create flashcards and practice quizzes. There are many pre-made sets of polyatomic ions on Quizlet, so you don’t have to create your own. Practice regularly and track your progress.
6. Draw Them Out:
Drawing the structure of the polyatomic ion can help you visualize it and remember it better. Practice drawing the ions and labeling the atoms and charges.
7. Memorize Common Prefixes and Suffixes:
Many polyatomic ions have common prefixes and suffixes that can help you remember them. For example, “per-” means “more than” and “ate” usually indicates a polyatomic ion with oxygen in it.
Related Article: How To Improve Your Study Skills? Best Tips And Techniques
FAQs:
Q1. What are some common polyatomic ions?
Some common polyatomic ions include sulfate (SO4 2-), nitrate (NO3 -),
Q2. How can I remember the charges of polyatomic ions?
One effective method is to use flashcards. Write the name of the ion on one side of the card and the charge on the other. Test yourself regularly and shuffle the cards to keep it challenging.
You can also use mnemonics and associations to help remember the charges. For example, phosphate (PO4 3-) has a charge of -3 because it has three oxygen atoms.
Q3. Can I memorize polyatomic ions without using mnemonics?
Yes, you can. Mnemonics are just one technique to help you remember polyatomic ions. You can also use flashcards, draw the structures, and practice with Quizlet to memorize them.
Q4. How many polyatomic ions do I need to know for chemistry class?
This can vary depending on your level of chemistry class. However, you should focus on learning the most common polyatomic ions first. These include sulfate (SO4 2-), nitrate (NO3 -), carbonate (CO3 2-), and phosphate (PO4 3-).
Q5. How can I apply my knowledge of polyatomic ions in real-life situations?
Understanding polyatomic ions is essential in chemistry and can be applied in various fields such as medicine, engineering, and environmental science. For example, understanding the role of nitrate (NO3 -) in the nitrogen cycle can help in agriculture and environmental management.
Q6. Can I use a song to memorize polyatomic ions?
Yes, you can. Many students find that setting the names and charges of polyatomic ions to a familiar tune can help them remember better. There are many polyatomic ion songs available online, or you can create your own.
Conclusion:
Memorizing polyatomic ions can be challenging, but with the right approach, it can be done effectively. By understanding the basics, focusing on common ions, using mnemonics, and practicing regularly, you can master polyatomic ions in no time.
Don’t forget to use mnemonics, flashcards, and Quizlet to help you memorize, and draw the structures to visualize them better. With these techniques, you’ll be able to recall the names and charges of polyatomic ions quickly and easily.